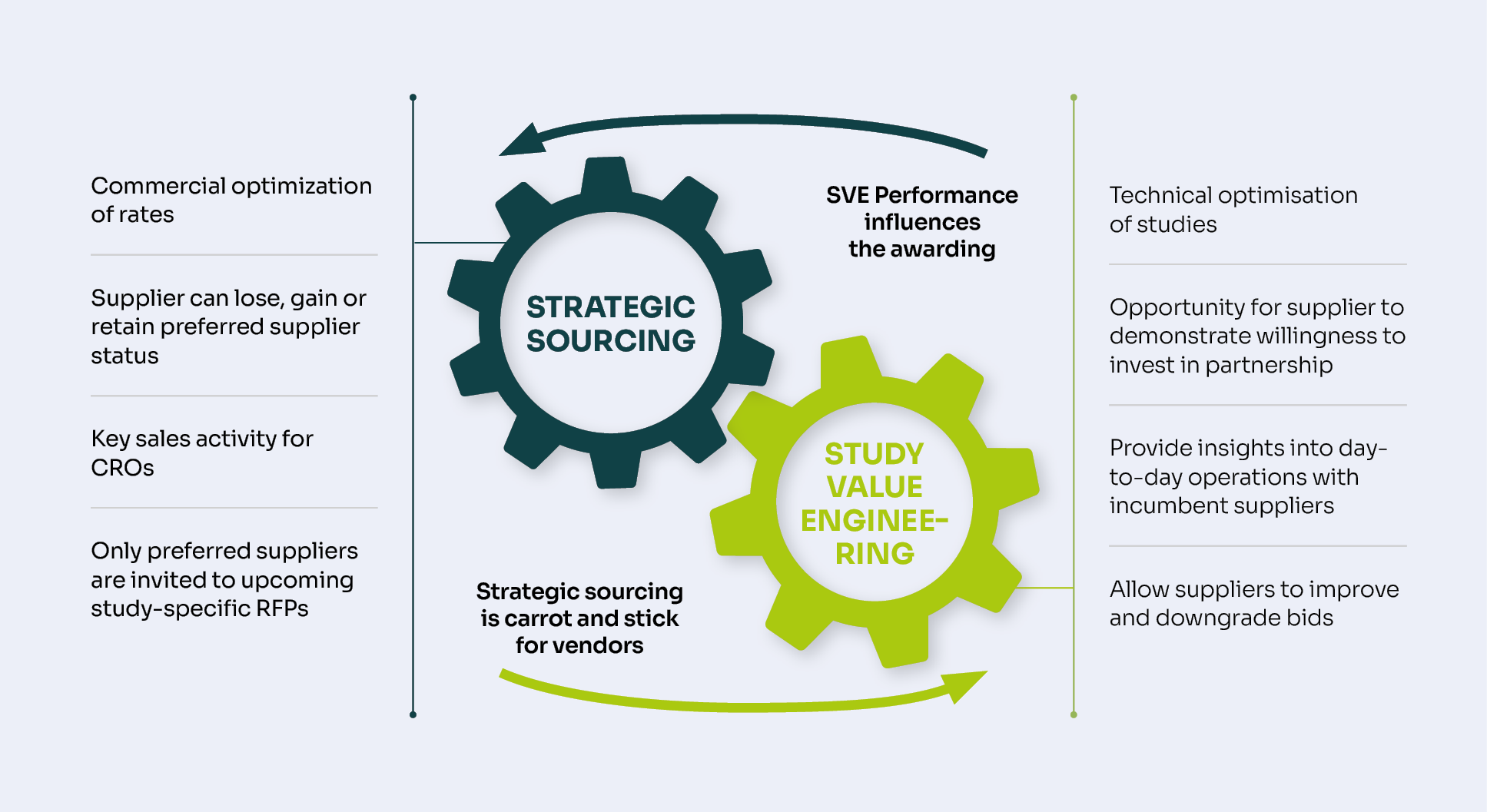
Bioassays: Provision of biological assays for evaluating the activity and efficacy of drug candidates
Cell line development: Supply and development of cell lines, including cloning services, drug testing and research
ADME/Toxicology/Pharmacology: Services related to drug ADME, toxicity studies, and pharmacology assessments
DNA/RNA synthesis & editing: DNA and RNA synthesis and editing services for molecular biology research in drug development
Protein sciences: Production, purification, and characterization of proteins, vital for drug target validation and research
Animal testing: Provision of animal models incl. species and strains relevant to specific diseases
Human Bio samples: Human bio. samples, such as tissues or fluids, necessary for studying drug effects and mechanisms
CMC & external MFG: Chemistry, Manufacturing and Controls (CMC), incl. external manufacturing of drug candidates to ensure quality & compliance of human bio samples (e.g., tissues or fluids, necessary for studying drug effects and mechanisms)
Other services: E.g., regulatory consulting, patient recruitment, clinical trial logistics, medical writing, bioanalytical services, etc.