Since its initiation in April 2024, the Critical Medicines Alliance (CMA) has made significant strides in addressing vulnerabilities in the supply of essential medicines and Active Pharmaceutical Ingredients (APIs). With the Strategic Report in its finalization phase and key discussions on the upcoming Critical Medicines Act already underway, the European Union is actively working to strengthen pharmaceutical supply resilience. These efforts come in response to ongoing supply shortages triggered by global disruptions such as the US-China trade war, the COVID-19 pandemic, and geopolitical tensions. While regulatory frameworks are taking shape, industry players must act now—reassessing their supply chain strategies proactively rather than waiting for legislative mandates.
The EU’s Derisking Strategy
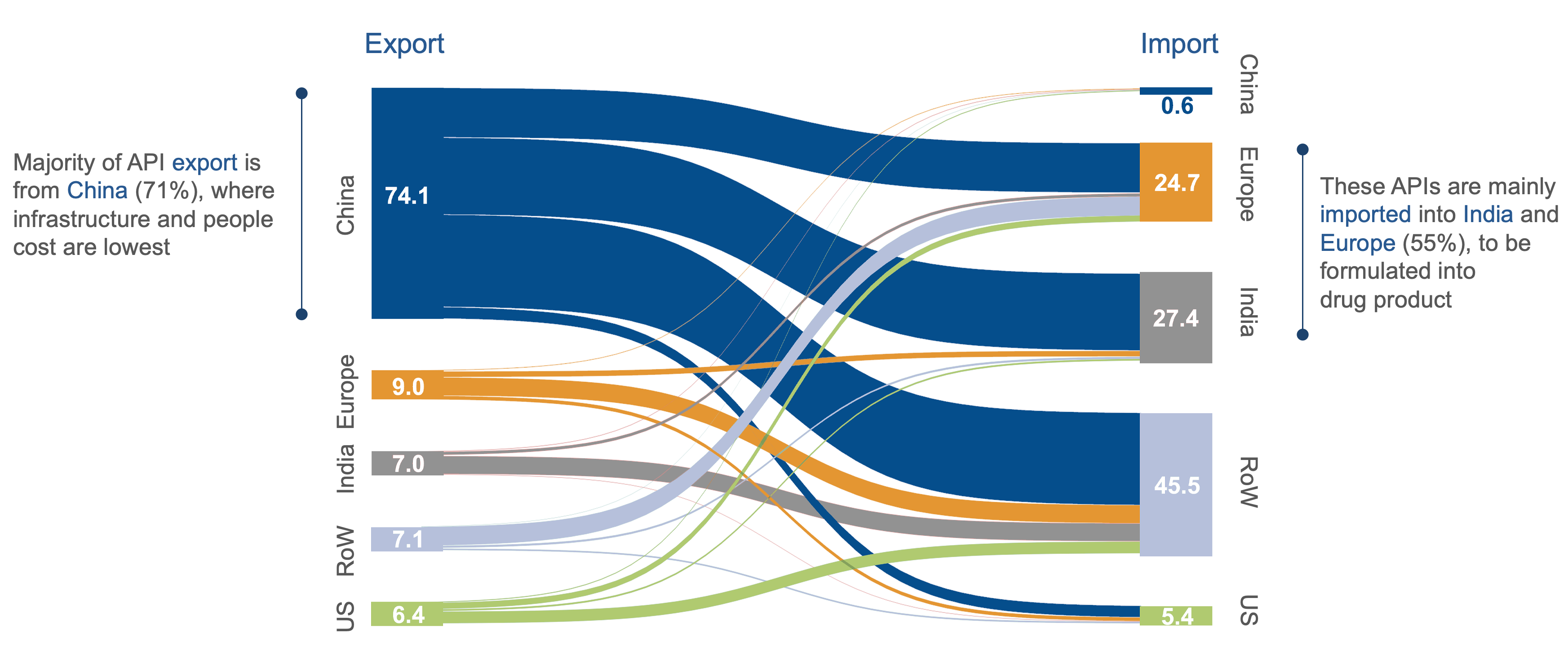
Source: IHS Markit (2020); BCG Expert interviews; API IHS Code – all 2941 H6 codes
The EU Medicines Alliance advocates a “derisking” strategy rather than complete decoupling from external API sources. This approach aims to reduce the EU’s dependence on API imports, particularly from regions like China and India, where combined, these countries represent nearly 70% of the sites manufacturing APIs for antibiotics in 2020.
Major Aspects Targeted by the Critical Medicines Alliance
The CMA focuses on several key areas to improve the pharmaceutical supply chain across Europe:
- Leverage EU and national funding to boost local manufacturing capabilities.
- Implement market incentives such as capacity reservation contracts and joint procurement to ensure a steady supply of critical medicines.
- Develop a Critical Medicines list to prioritize the availability of essential treatments and APIs identified at risk.
- Implement a European Voluntary Solidarity mechanism to streamline the distribution of critical medicines during shortages.
- Introduce regulatory adjustments to speed up access to crucial medicines without compromising safety.
- Foster strategic autonomy by reducing dependence on non-EU sources for APIs and other critical raw materials.
- Facilitate cooperation between the European Health Emergency Preparedness and Response Authority (HERA), the European Medicines Agency (EMA), industry, civil society, and governmental bodies.
- Coordinate EU-wide procurement strategies to secure essential treatments across member states.
How pharmaceutical companies can become more resilient
To navigate the evolving landscape and anticipate future regulations, companies first have to become aware of the risk (levels) along the standard pharmaceutical supply chain. As they cross the globe multiple times, they are at highly at risk due to sourcing countries.
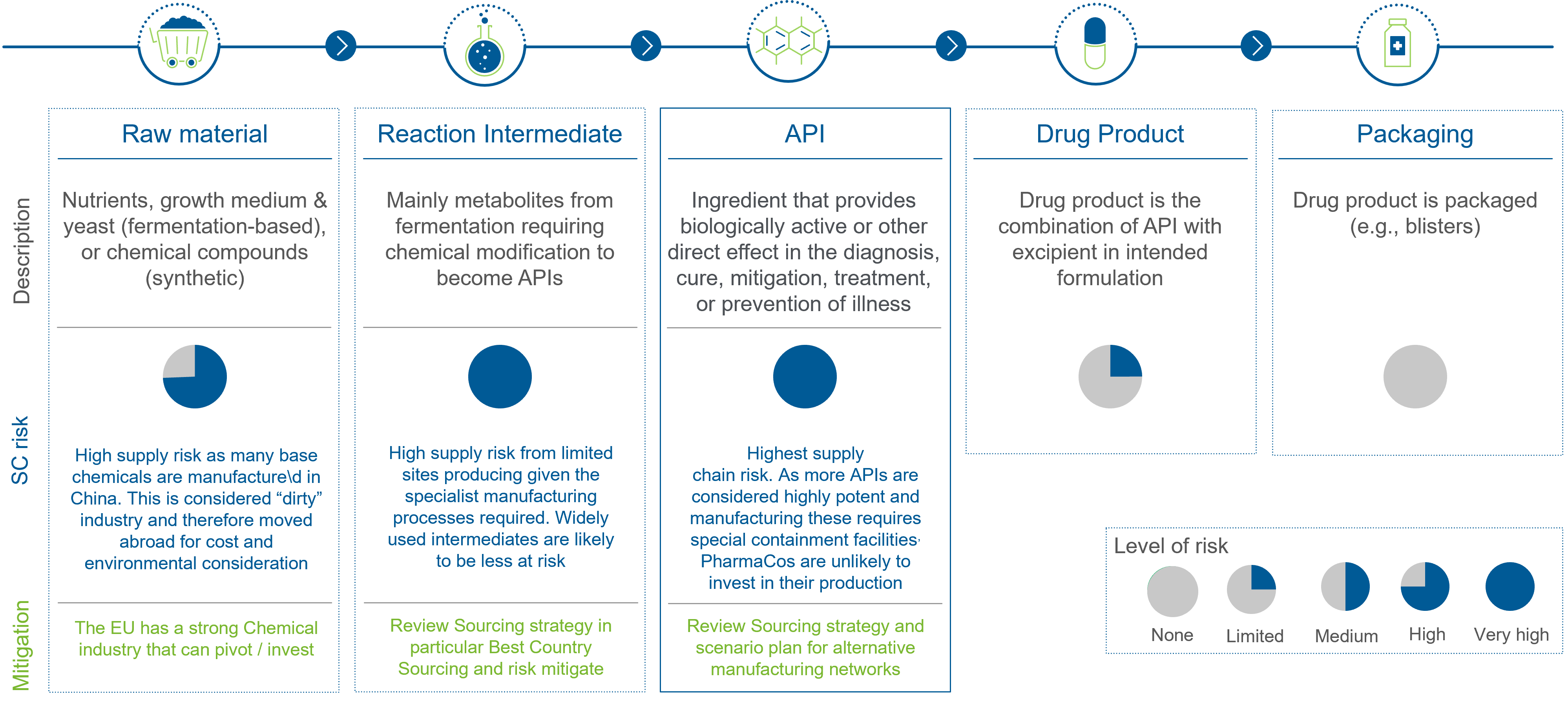
See more details on the EU Critical Medicines Alliance and its progress here.
Pharmaceutical players can mitigate their risks with a 3-step-approach
-
Supply chain diagnostic and resilience review
- Conduct thorough diagnostics to identify vulnerabilities such as regional dependencies and lack of supplier diversification.
- Optimize inventory management and develop alternative supply scenarios, including nearshoring possibilities.
-
Rethink your sourcing strategy
- Include multiple award contracts and integrate supply security into tender evaluations.
- Promote cross-functional collaboration to adapt specifications and minimize the impact of supply constraints.
-
Embrace strategic collaboration
- Actively engage with the Critical Medicines Alliance to explore joint procurement and other collaborative opportunities.
- Utilize strategic collaborations to enhance supply security and operational resilience.


